Biologics discovery
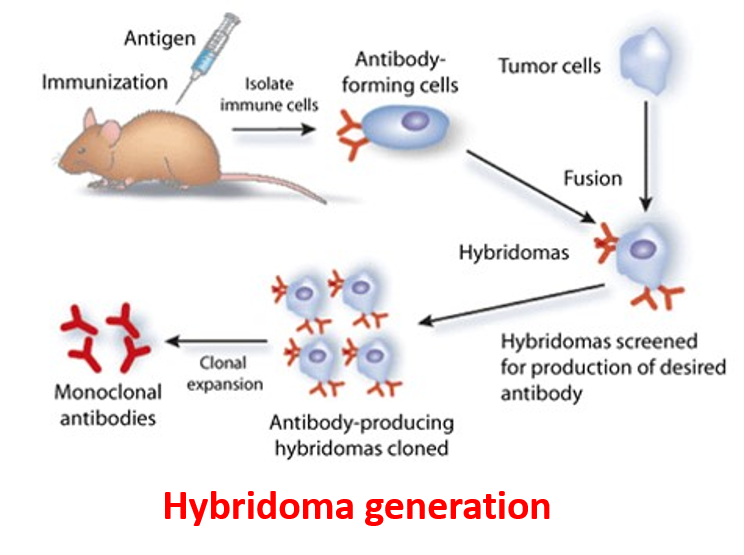
Eviogen offers a diversity of immunization schemes and immunogens. Immunizations are performed at our onsite vivarium with instant access to different types of hosts like mice, rats and hamsters. We use variant strains of mice for higher epitope diversity. Our scientific staff has extensive experience with different routes of administration using a variety of adjuvants.
- Development of Monoclonal and polyclonal Antibodies.
- We Take development of high-quality antibodies against therapeutic Proteins, Peptides and Small Molecules.
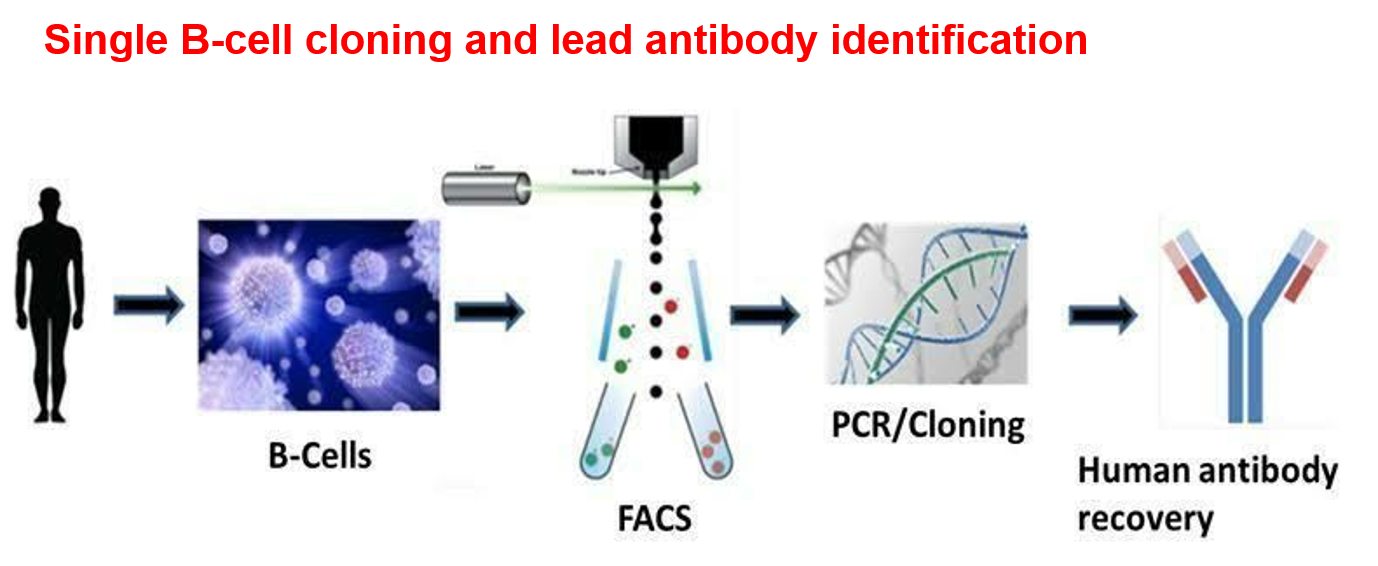
- Development and validation of Method for Neutralizing Antibodies. Single B-cell cloning .
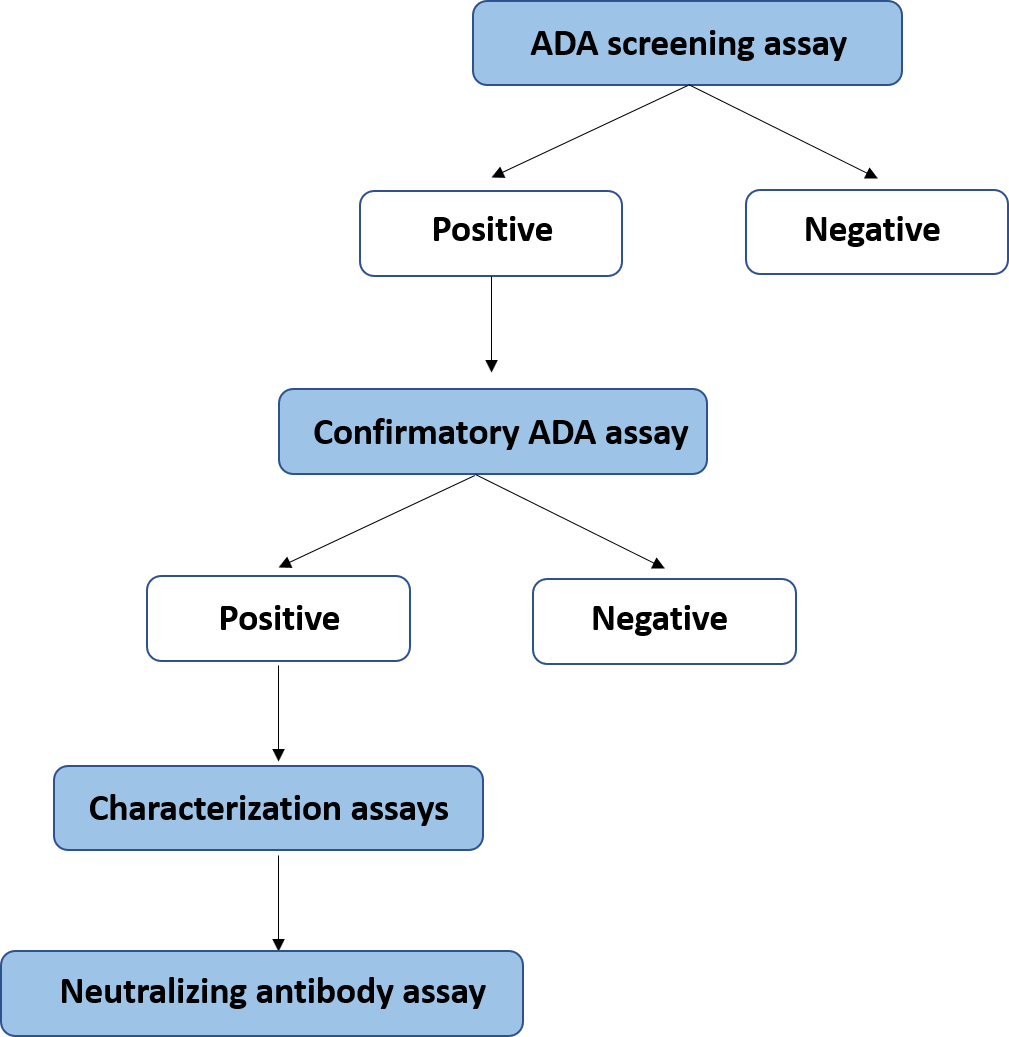
Immunogenicity assays:
- Development and Validation of Method according to EMEA and FDA guidelines.
- Analysis of Samples in GLP controlled environment.
- Development and validation of Method for Neutralizing Antibodies.
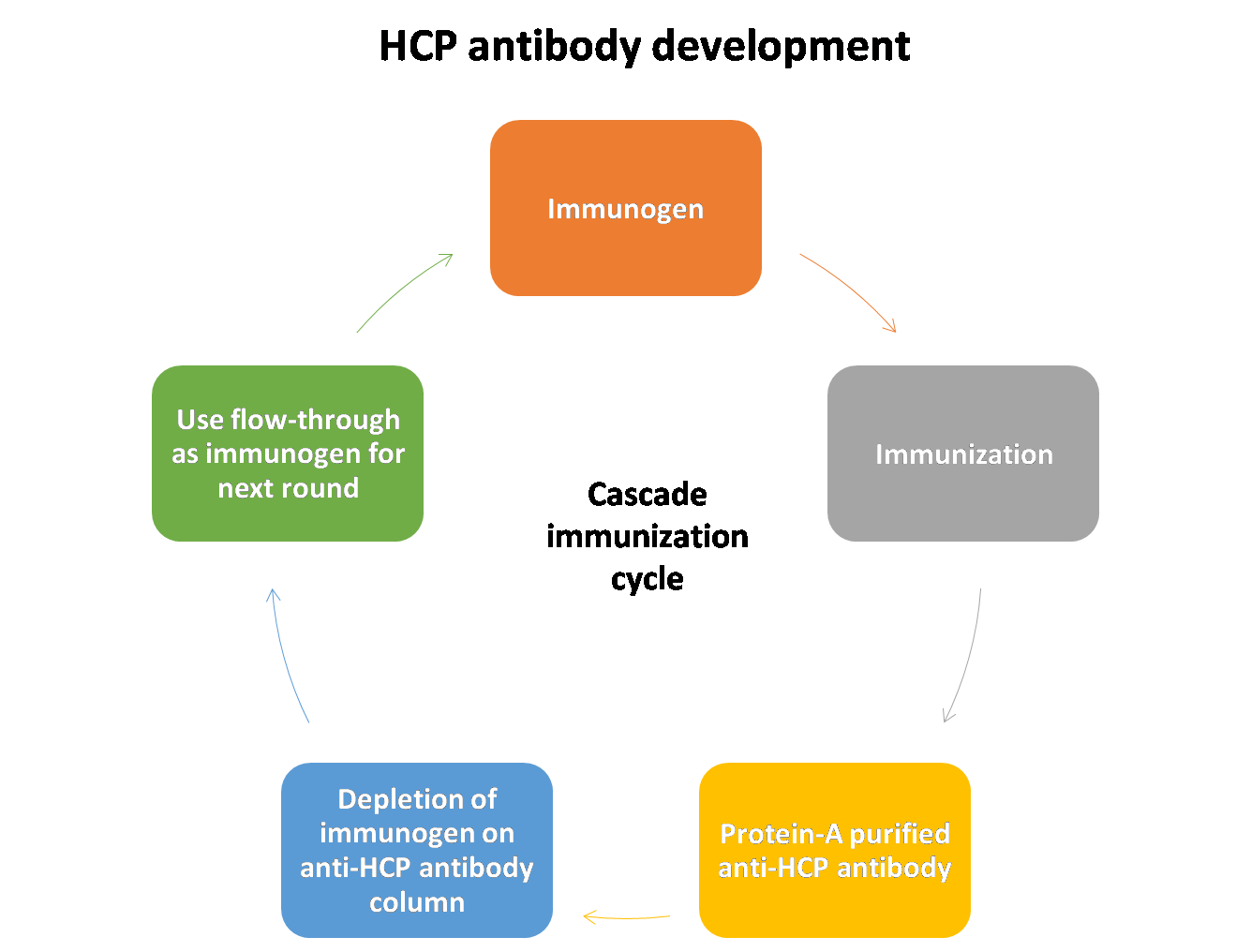
Development and Validation of Host Cell Protein Assay:
Host-cell proteins (HCPs) constitute a major part of process-related impurities during biologics production. The amount of residual HCPs in drug product is generally considered a critical quality attribute (CQA), due to their potential to affect product safety and efficacy. Therefore, it is a regulatory requirement to monitor the removal of HCPs in drug product during bioprocess development.
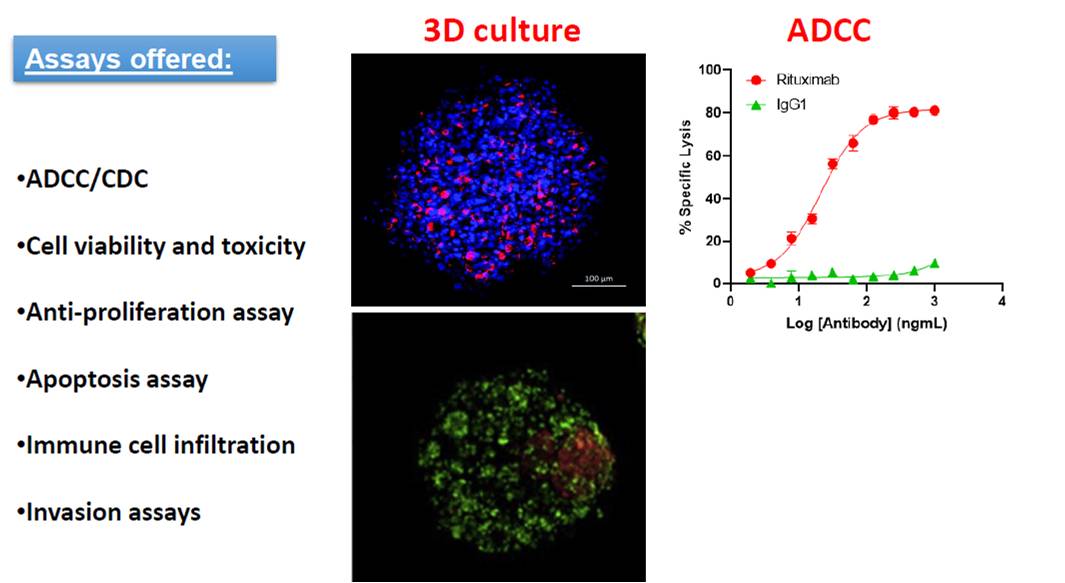
ADCC/CDC, 3D culture and co-culture assays
Eviogen specializes in assays for the screening of anti-cancer drugs. We offer assays formats using traditional monolayer models, as well as 3D cell culture models, which better recapitulates the tumor microenvironment in vivo